Scientists in Tennessee claim that, somewhat serendipitously, they converted carbon dioxide into ethanol.
The researchers, who work at the Department of Energy’s Oak Ridge National Laboratory, developed a process that adds “nano-spikes” – essentially tiny bursts – of carbon and copper to CO2 to transform it into ethanol, the type of alcohol found in hand sanitizer and alcoholic drinks.
Ethanol can also be turned into fuel – gasoline in Brazil contains more than 25% ethanol – which is why the scientists are calling the discovery a “twist to waste-to-fuel technology.”
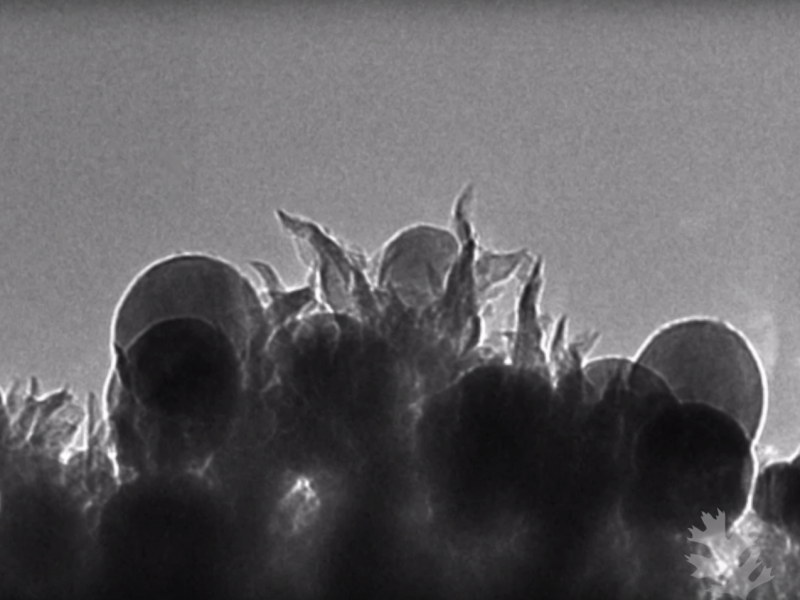
"We discovered somewhat by accident that this material worked," Adam Rondinone, lead author of the study, said in a press release. "We were trying to study the first step of a proposed reaction when we realized that the catalyst was doing the entire reaction on its own."
The team's experiment was meant to be one part of a longer research project investigating how to turn CO2 into ethanol; the researchers figured the process would require multiple steps and complicated chemical reactions. But it turned out to be a lot easier than they thought: They needed only a single catalyst (copper) to transform the CO2.
The discovery is a major breakthrough, considering the process turns carbon dioxide - one of the air pollutants contributing to climate change - into fuel, which in turn generates more CO2 that could be turned into more fuel. (Burning a gallon of diesel fuel produces about 22 pounds of CO2.)
If the technology becomes cost-efficient and widely available, it could provide a new carbon-neutral alternative to fossil fuel production.
There's no word yet on whether the discovery will leave the lab, however.
Watch the scientists explain:











