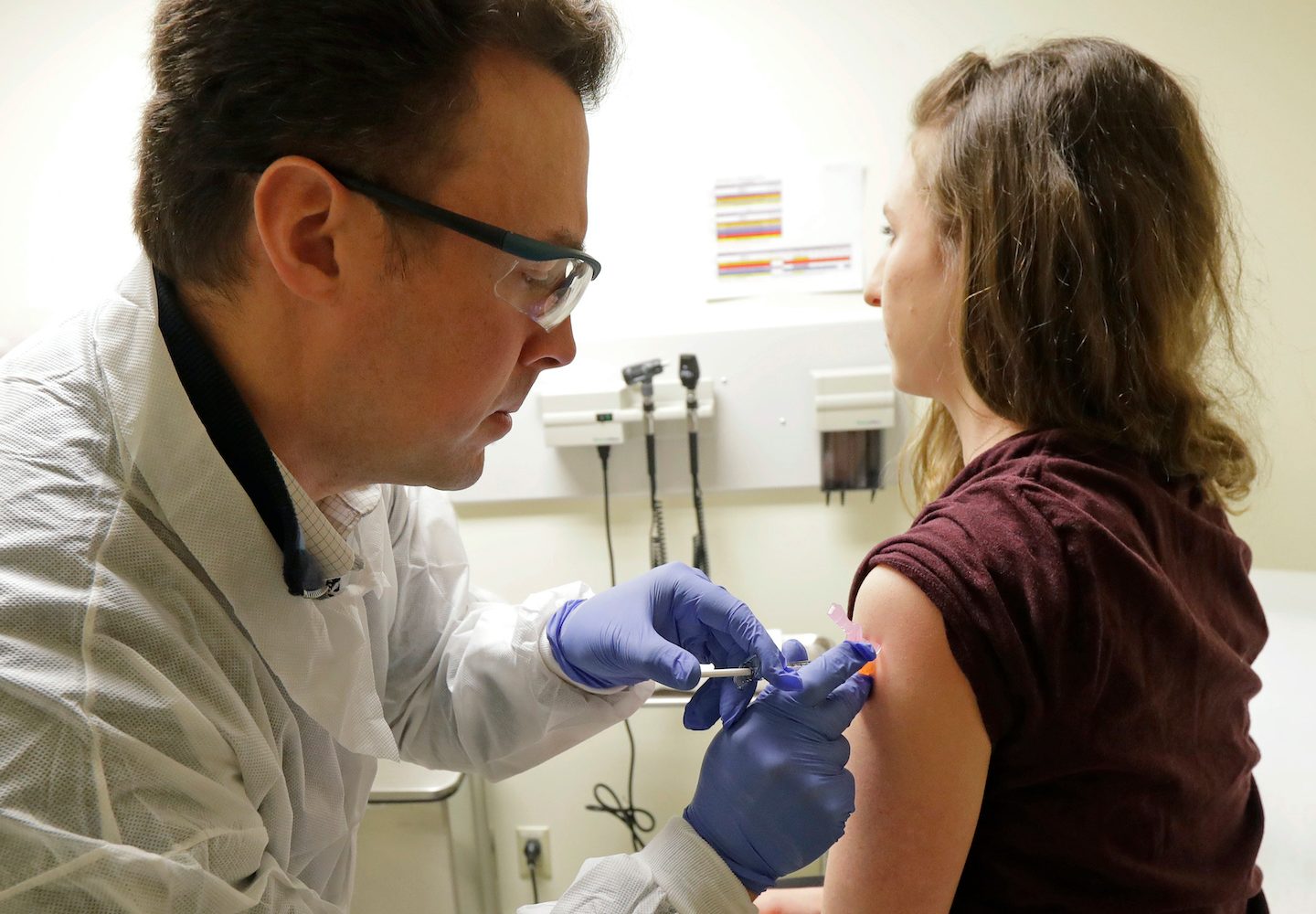
- The first human clinical trial of a COVID-19 vaccine was administered Monday.
- Four volunteers were given their first of two shots of the vaccine. Forty-five volunteers are expected to participate in this trial.
- The vaccine won’t be available to the general public for at least a year as it’s tested for safety and efficacy.
- The novel coronavirus, which causes the infectious disease known as COVID-19, has infected more than 182,000 people worldwide and killed more than 7,100.
- Visit Business Insider’s homepage for more stories.
Four healthy people were the first to get a trial COVID-19 vaccine on Monday.
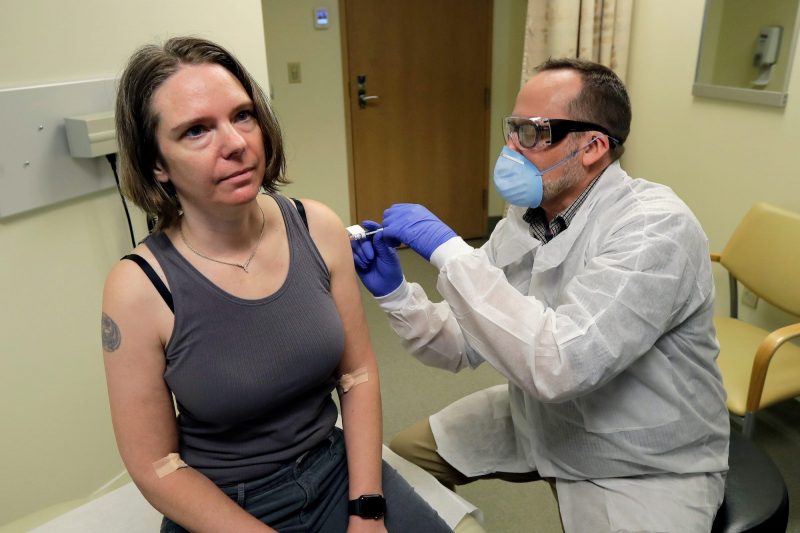
Foto: A pharmacist gives Jennifer Haller the first shot in the first-stage safety study clinical trial of a vaccine for COVID-19. Source: AP Photo/Ted S. Warren
Source: The Associated Press
The vaccine was administered to patients at the Kaiser Permanente Washington Health Research Institute in Seattle.

Foto: A syringe containing the first shot given in the trial. Source: AP Photo/Ted S. Warren
Source: The Associated Press
Forty-five volunteers will be part of the clinical trial. The volunteers who were selected were screened by Kaiser to not be sick nor have underlying health conditions. They were not screened, however, for mild cases of COVID-19.

Foto: Rebecca Sirull gets a shot of the new trial vaccine for COVID-19. Source: AP Photo/Ted S. Warren
Source: Business Insider, AP
It will take at least a year to 18 months to determine whether a vaccine for the novel coronavirus is safe and effective, National Institutes of Health experts have said.

Foto: Haller waits for her vaccine shot. Source: AP Photo/Ted S. Warren
Source: Business Insider
To see which dosage is most effective, some of the participants may get a higher dosage of the vaccine.

Foto: Witte opens a frozen package of the trial vaccine for COVID-19. Source: AP Photo/Ted S. Warren
Source: AP
Volunteers will be given two doses. The second dose will be administered a month after the first.
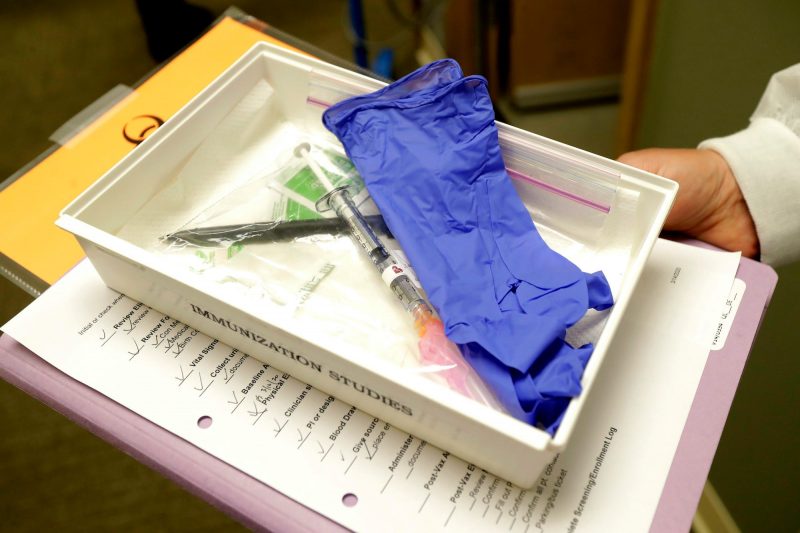
Foto: A tray with a syringe containing a shot that will be used in the clinical trial of the COVID-19 vaccine. Source: AP Photo/Ted S. Warren
Source: AP
This vaccine was developed by the National Institutes of Health and the private biotech company Moderna.

Foto: Witte takes a package of the vaccine out of a freezer. Source: AP Photo/Ted S. Warren
Source: AP
The vaccine was created in 42 days.

Foto: Neal Browning gets a shot of the vaccine. Source: AP Photo/Ted S. Warren
Source: Business Insider
The test vaccine works by producing harmless "spike" proteins meant to prompt the body to create antibodies. The idea is that if a person were to be exposed to the real coronavirus, their body would be prepared to react quickly, as spike proteins are what allow the novel coronavirus to attach themselves to human cells.

Foto: Witte opens a package that contains the trial vaccine for COVID-19. Source: AP Photo/Ted S. Warren
Source: AP
If approved, it would be first to use just the virus' "messenger RNA" sequence and not the virus itself. The vaccine won't infect participants with the coronavirus that causes COVID-19.
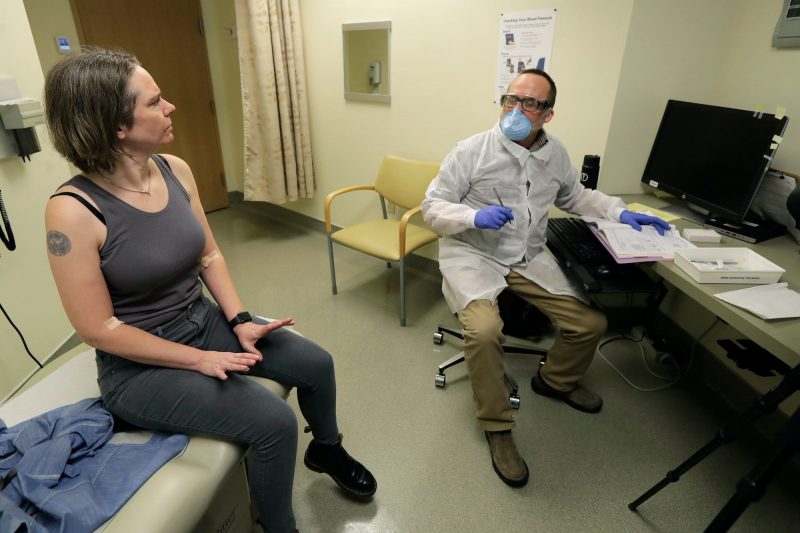
Foto: Haller talks to a pharmacist after getting her first shot. Source: AP Photo/Ted S. Warren
Source: Business Insider, AP
While participants don't risk getting the virus, the safety of the vaccine is still unknown. Scientists don't know how the immune system will respond to the test.

Foto: Pharmacists talk after prepping to administer the vaccine trial. Source: AP Photo/Ted S. Warren
Source: AP
While this is the first of the COVID-19 vaccines to make it to a clinical human trial, other vaccines are also underway. Before any vaccine becomes available to the general public, researchers have to be sure it is safe and effective.

Foto: Vials used by pharmacists to prepare syringes used on the first day of a first-stage safety study clinical trial of the potential vaccine for COVID-19. Source: AP Photo/Ted S. Warren
Source: Business Insider, AP