EJ Hersom, Department of Defense
- The Department of Defense released images of the paper cards Americans will receive to keep track of COVID-19 vaccine doses.
- Vaccines from Moderna and Pfizer require two separate doses, taken either three or four weeks apart, respectively.
- If taken correctly, preliminary analysis from late-stage trial data suggests the vaccines will be highly effective at protecting against COVID-19.
- An independent advisory committee will meet on December 10 to decide whether to recommend Pfizer’s vaccine for FDA emergency authorized use.
- Visit Business Insider’s homepage for more stories.
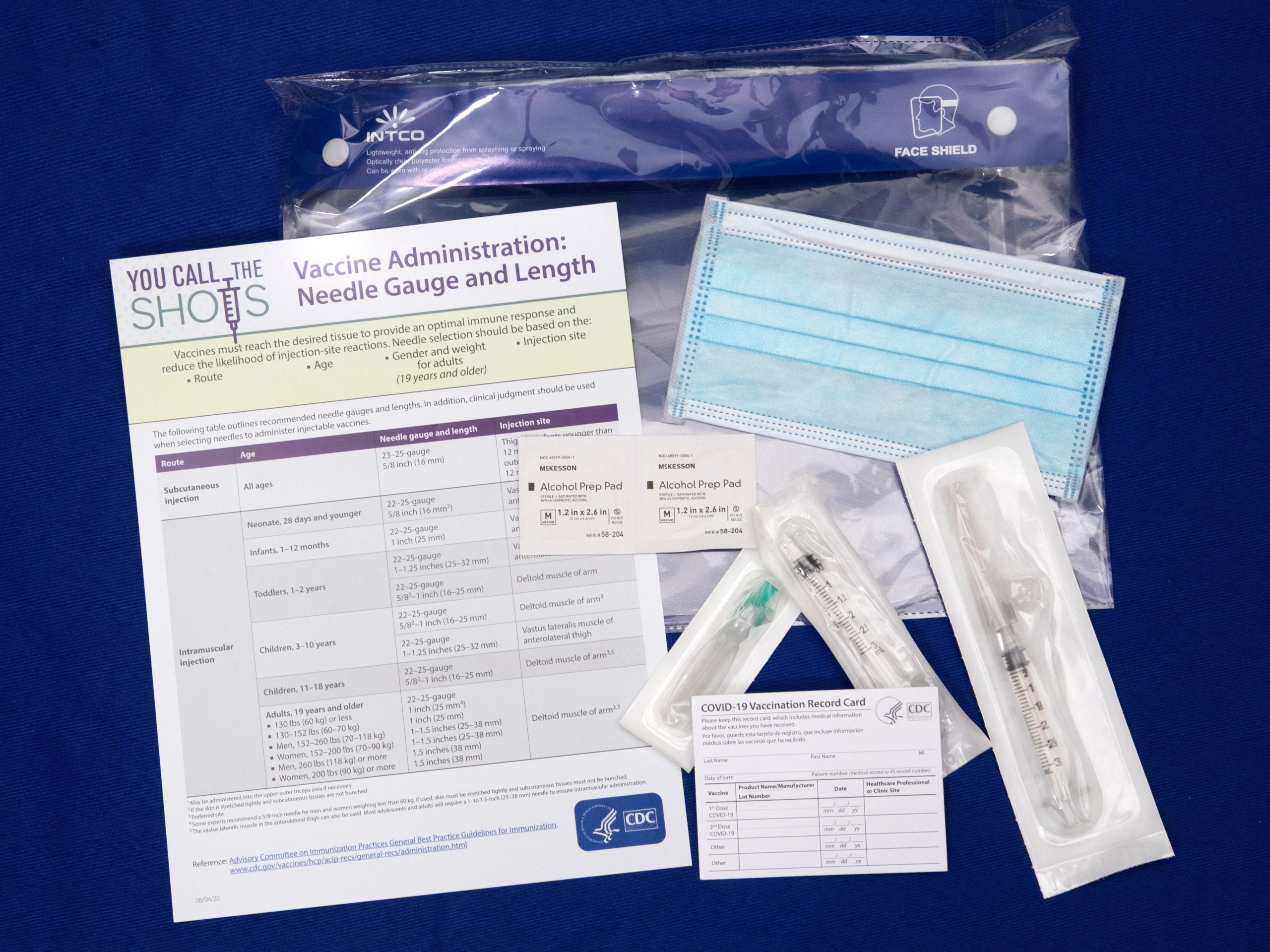
The US will give you paper cards to keep track of when to get your COVID-19 vaccine.
Vaccines developed by Pfizer and Moderna, which are both awaiting a green light for emergency authorized use, require two doses. Recipients must take Pfizer’s second vaccine dose four weeks after the initial one, and Moderna’s three weeks after.
To remind Americans to get both vaccine doses, Army Gen. Gustave Perna said Wednesday the US government will provide doctors’ offices with ancillary kits that contain paper cards, as well as needles and syringes for the vaccines. Images of the cards, taken by the Department of Defense, indicate recipients will manually log when and where they got the first vaccine dose.
The cards will be issued in English, with some information in Spanish.

EJ Hersom, Department of Defense
If taken correctly, preliminary analysis from late-stage trial data suggests the vaccines will be highly effective at protecting against COVID-19. Pfizer’s vaccine was 95% effective at preventing volunteers against COVID-19, while Moderna’s was 94.1% effective.
An independent advisory committee will meet December 10 to assess whether the Pfizer vaccine is fit for FDA approval. The FDA will likely decide on Moderna's vaccine after Pfizer's.
Operation Warp Speed, the US government's agency leading the vaccine effort, have funded research for other developers, including Johnson & Johnson, AstraZeneca and the University of Oxford, Novavax, and Merck. Johnson & Johnson is still conducting trials, while AstraZeneca and the University of Oxford will likely retest its vaccine after scientists criticized the initial data.
Moncef Slaoui, the chief advisor of Operation Warp Speed, said the US aims to have 20 million people vaccinated by the end of the year. Perna said the Defense department will begin shipping the vaccine within 24 hours of an FDA greenlight.
Cases, hospitalizations, and deaths from COVID-19 reached record levels in the last few weeks during a third surge of the disease. Nearly 275,000 Americans have died due to COVID-19, and almost 14 million have been infected so far.